Rewind: Deadly cough cure — how safe are our medicines
A series of child deaths linked to contaminated cough syrup has exposed deep cracks in India’s drug regulation and manufacturing oversight
By Kattamreddy Ananth Rupesh
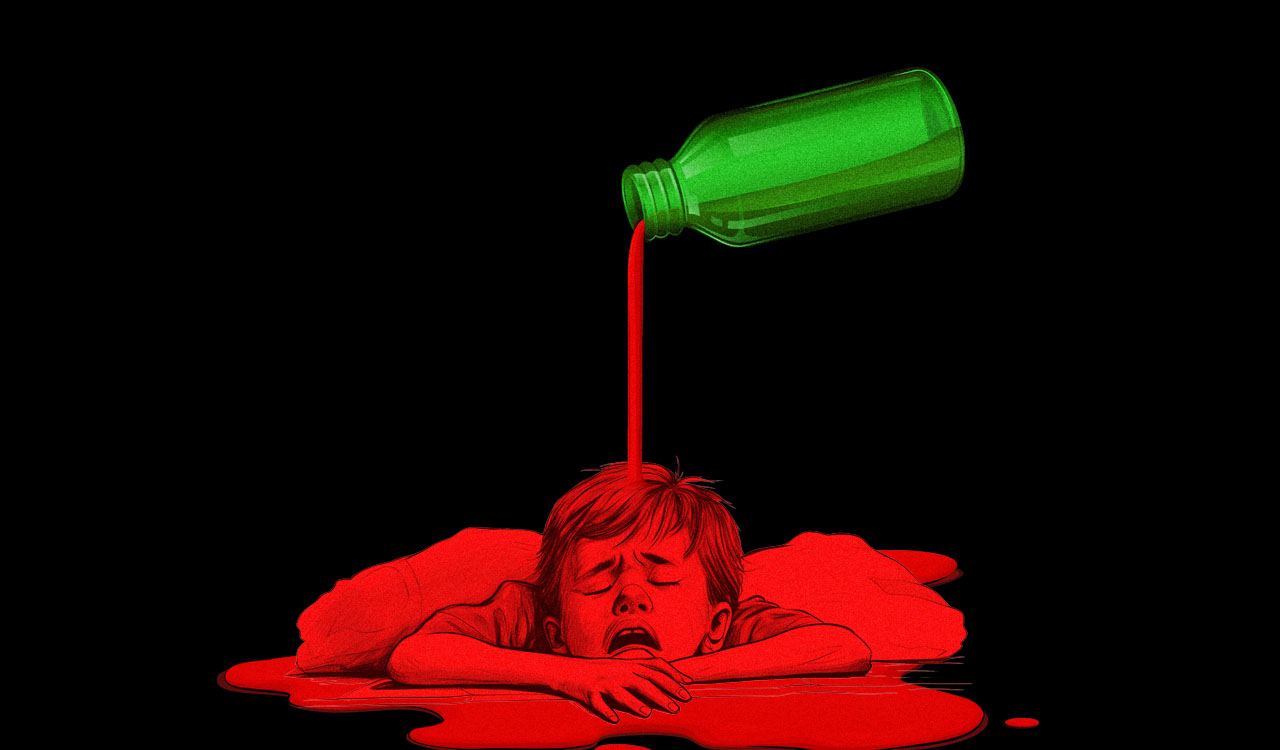
In the recent past, at least 14 paediatric deaths have been allegedly linked to contaminated cough syrups (Coldrif: Paracetamol, Phenylephrine and Chlorpheniramine) in Madhya Pradesh and Rajasthan. While initial conflicting reports suggested that the samples were free of ethylene glycol (EG) and diethylene glycol (DEG), subsequent tests conducted by the Tamil Nadu Drug Controller at the manufacturing unit revealed DEG levels 480 times higher than the permissible limit in the medicines purportedly associated with the unnatural deaths of children.
Also Read
A multidisciplinary team, including experts from the National Institute of Virology (NIV), National Environmental Engineering Research Institute (NEERI), Central Drugs Standard Control Organisation (CDSCO), National Centre for Disease Control (NCDC), and the All India Institute of Medical Sciences (AIIMS), Nagpur, has been constituted to examine the matter. The team is tasked with analysing multiple samples and investigating factors related to the reported paediatric deaths. Their work includes reviewing clinical records, evaluating possible infectious causes, assessing environmental and entomological factors, and examining autopsy findings and postmortem toxicology reports to gather evidence linking the syrup to the deaths.
In response, the Directorate General of Health Services (DGHS) issued an advisory to all States and Union Territories, recommending that cough and cold medications should not be prescribed or dispensed to children under two years of age. For children above two, the advisory notes that such medications are generally not recommended for those under five and should be used only after careful clinical evaluation, close supervision, strict adherence to dosage, and avoidance of multiple drug combinations. The DGHS also emphasised non-pharmacological measures, including adequate hydration, rest, and supportive care, as the first-line management for acute cough illnesses in children.
Fatal Formula
- 14 child deaths in India linked to diethylene glycol (DEG)-tainted cough syrup highlight regulatory gaps
- DEG levels found 480 times above limit at a licensed manufacturing facility
- 25 global incidents in 90 years, over 1,300 lives lost, mostly children
- Primarily used as an antifreeze and a component of hydraulic brake fluid, DEG is a highly toxic industrial solvent
Following these developments, the Health Ministry convened an emergency, high-level meeting with State health secretaries and drug control officials. The discussion focused on stricter oversight of cough syrup production, rational prescribing practices (especially in children), and the expansion of drug safety surveillance mechanisms. Officials stressed the need for full compliance with the Revised Schedule M (GMP standards) of the Drugs and Cosmetics Act, warned of licence cancellations for non-compliance, and urged inter-State coordination for early detection and response to safety breaches. Several States have since prohibited the sale of Coldrif.
Toxic Mixture
DEG is an effective solvent for water-insoluble substances and has a sweet taste, leading to its use — both inadvertent and deliberate — as a diluent or substitute for glycerine or propylene glycol in pharmaceutical formulations. Historically, DEG has also been illicitly added to wines as a sweetening agent, leading to mass poisonings. Its deceptive sweetness and low cost make it a particularly dangerous adulterant.
Primarily used as an antifreeze and a component of hydraulic brake fluid, DEG is a highly toxic industrial solvent. Even small quantities can cause acute kidney failure, metabolic acidosis, neurological damage, and death. The estimated fatal dose in adults is typically less than 100 ml, with children being especially vulnerable due to their underdeveloped liver and kidney detoxification mechanisms. Symptoms usually appear within a few hours, beginning with nausea, vomiting, and abdominal pain, followed by renal failure, central nervous system depression, and metabolic disturbances. Without prompt medical treatment, death may occur within 24-72 hours from multi-organ failure.
Mass Poisonings
Excipients like glycerine, sorbitol, propylene glycol, and maltitol are common in syrups and personal care products. However, contamination with EG and DEG has repeatedly caused mass poisonings globally. According to a joint World Health Organization (WHO) and the United Nations Office on Drugs and Crime (UNODC) report, contamination of pharmaceutical excipients represents a serious global health concern.
Over the past 90 years, at least 25 documented incidents of excipient contamination, such as in tainted paracetamol syrups, have resulted in more than 1,300 deaths worldwide, many of them among children. These tragedies have disproportionately affected low- and middle-income countries, where regulatory oversight and access to quality-assured medicines are limited.
Since the 1937 Massengill disaster in the United States, which killed 105 people, similar DEG contamination cases have occurred in Panama, China, Haiti, Bangladesh, Argentina, and Nigeria. The Massengill tragedy led to the US Food, Drug, and Cosmetic Act of 1938, establishing one of the world’s most stringent pharmaceutical safety regulations. Yet, such incidents persist — a collective failure of global health safeguards.
More recent outbreaks in Uzbekistan, The Gambia, and Indonesia have claimed over 250 lives, mostly among children under five. These incidents highlight vulnerabilities in the global pharmaceutical supply chain and the urgent need for stronger international safeguards, regulatory vigilance, and accountability.
The 2022 Gambia case, involving medicines manufactured in India, led to around 70 child deaths and exposed serious lapses in quality control and regulatory oversight. Allegations of sample manipulation and corruption further underscored the need for transparency and global collaboration among drug regulators.
India and Pakistan have also faced similar crises since the late 1980s, with adulterated cough syrups and contaminated acetaminophen formulations causing multiple fatalities. These events have tarnished the reputation of India’s pharmaceutical sector and raised serious doubts about adherence to Good Manufacturing Practices (GMP).
Inadequate Oversight
The Drugs and Cosmetics Act empowers both central and State authorities to regulate drug manufacturing and sales, with the Central Drugs Standard Control Organisation (CDSCO) overseeing approvals, clinical trials, and imports, and State Drug Regulatory Authorities (SDRAs) monitoring domestic production and distribution. The Indian Pharmacopoeia (IP) sets official safety standards.
Although the Drugs and Cosmetics Act has been foundational in establishing legal standards for safety and efficacy, it is increasingly viewed as outdated. A key structural weakness lies in the division of responsibilities — while the Centre handles approvals and licensing, enforcement and quality monitoring largely rests with the States, many of which lack adequate infrastructure, trained staff, or laboratories. This results in uneven compliance and enforcement.
Another serious concern is weak deterrence and enforcement. Although the Act provides for penalties, including imprisonment for manufacturing spurious or substandard drugs, in practice, many cases result in minor sanctions such as temporary licence suspensions, modest fines, or delayed prosecution. Root‐cause analyses, legally required in many cases, are seldom carried out. By contrast, regulators such as the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) enforce stricter oversight, transparent approvals, and robust post-market surveillance.
Class-action suits, common in Western nations, are rare in India. While criminal prosecution is possible under the Drugs and Cosmetics Act, it is often difficult due to cumbersome procedures and the high burden of proof. The Jan Vishwas (Amendment of Provisions) Act, 2023, further decriminalises several offences, replacing imprisonment with fines — thereby reducing deterrence. The pharmaceutical sector is often viewed more as a business enterprise than a public health responsibility. Manufacturers have illegally used EG and DEG as substitutes for glycerine or propylene glycol, exploiting their similar physicochemical properties to cut costs.
Tragedy Repeats
- The Massengill disaster of 1937, which killed 105 Americans, led to the creation of the US Food, Drug, and Cosmetic Act of 1938
- Yet, nearly 90 years later, developing countries are still losing children to the same poison
- The tragedies in The Gambia, Indonesia, and Uzbekistan involve exported drugs manufactured in India
As Pawan Kumar et al noted in the Regulatory Toxicology and Pharmacology journal, the issue boils down to mismanagement of the pharmaceutical raw material supply chain. Weaker supplier verification, poor quality control, and unclear ingredient accountability (confusion over who is responsible for checking the safety of ingredients before they enter production) create systemic vulnerabilities and erode public trust.
Following the Gambia incident, the government updated excipient pharmacopoeia standards to align with international guidelines and strengthened surveillance, including setting limits for EG and DEG at Not More Than (NMT) 0.10%. More stringent measures are still needed.
Currently, Thin Layer Chromatography (TLC) is used for DEG/EG screening in excipients, while confirmatory quantitative testing is performed using advanced methods like GC-MS at accredited laboratories. The next step must include mandatory testing of all high-risk excipients, especially from non-authorised sources, and the development of portable, rapid, and cost-effective detection tools.
Trust Prescription
The Indian Pharmacopoeia Commission recommends a triple-pronged strategy:
- Regulators must enforce guidelines, conduct risk-based inspections, ensure transparent reporting, and take swift corrective and criminal action.
- Manufacturers must adhere to GMP, ensure supply chain integrity, train personnel to prevent contamination, and invest in infrastructure to detect impurities.
- Consumers and healthcare professionals must choose brands with a proven track record of safety, report adverse effects, and stay informed about drug purity and safety standards. Additionally, raising awareness among doctors and parents on the rational use of medicines is essential to prevent unnecessary prescriptions and adverse outcomes.
A growing menace is the sale of falsified drug excipients through e-commerce platforms, which often evade regulatory checks. The WHO-UNODC report warns that organised criminal groups exploit digital markets, falsify documentation, and introduce industrial-grade chemicals into global supply chains. Hence, beyond regulation, criminal justice mechanisms are vital.
India’s regulatory failures risk not only domestic health but also global trust. While export medicines undergo scrutiny, those for domestic use often face lax oversight, allowing substandard and counterfeit drugs to circulate. Recent cases, such as the seizure of fake anti-cancer drugs in Telangana, reveal the magnitude of the problem.
Moreover, the lack of active engagement by doctors in raising concerns about substandard medicines is adding to the problem. The menace of quackery has already worsened antimicrobial resistance.
The toxic nexus between certain segments of the pharmaceutical industry and healthcare providers must be addressed to prevent the over-promotion of proprietary medicines. Over-prescription has become common, often driven by patient-related pressures.
Product liability standards for both medical devices and drugs must be set at a high level to safeguard public health and ensure accountability across the industry. The loss of innocent children to contaminated syrups is a national tragedy. This heartbreaking episode calls for immediate accountability and stronger protections to safeguard our most vulnerable.
(The author is Assistant Professor of Forensic Medicine, Government Medical College, Ongole)
Related News
-
TG EAPCET 2026: JNTU-Hyderabad freezes test zones in five districts
1 min ago -
Austria women’s hockey team arrives in Hyderabad for FIH World Cup Qualifiers
12 mins ago -
Nirmal Collector inspects KGBV, instructs officials to raise education standards
20 mins ago -
108,000 US troops in 160 countries, Generals tell Senators
21 mins ago -
BC aspirants get a raw deal in Congress Rajya Sabha nominations
29 mins ago -
Sarpanch’s husband attacked with boiling water during Holi in Khammam
39 mins ago -
New Iranian attacks target Israel and US bases as more Israeli strikes hit Lebanon
44 mins ago -
Iran’s foreign minister says US ‘will come to bitterly regret’ precedent set after sub sank frigate
45 mins ago




