TSDCA conducts raids, seizes unlicensed medical shops across Telangana
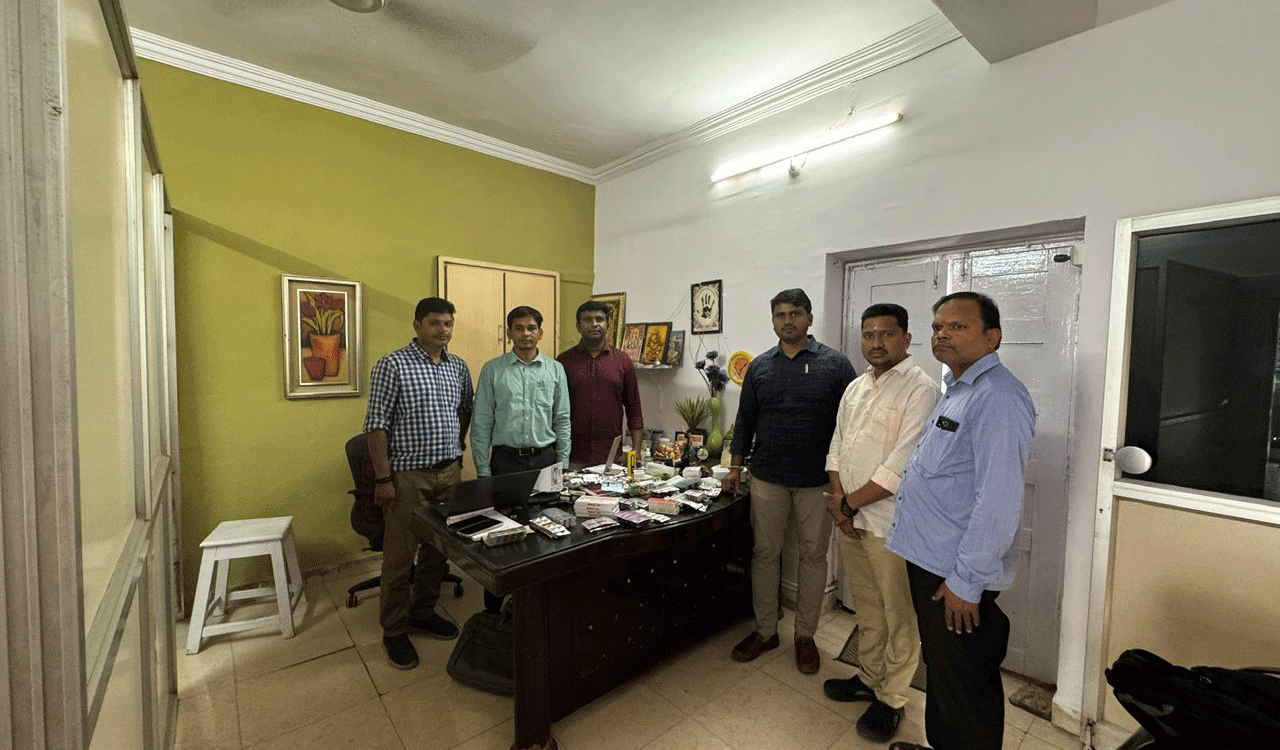
During the course of the raids, the TSDCA seized 23 varieties of medicines including antibiotics, analgesics etc worth Rs 18, 000 from the illegally run medical shop
Hyderabad: The Telangana State Drug Control Administration (TSDCA) conducted raids at an unlicensed medical shop in Komatwadi, Noorkhan Bazar, Charminar, being run by Dr Yahya Aslam Bin Mahfooz illegally without a valid drug license.
During the course of the raids, the TSDCA seized 23 varieties of medicines including antibiotics, analgesics etc worth Rs 18, 000 from the illegally run medical shop.
Stocking drugs for sale without a drug license is punishable under the Drugs and Cosmetics Act, with imprisonment for up to five years, the TSDCA officials said.
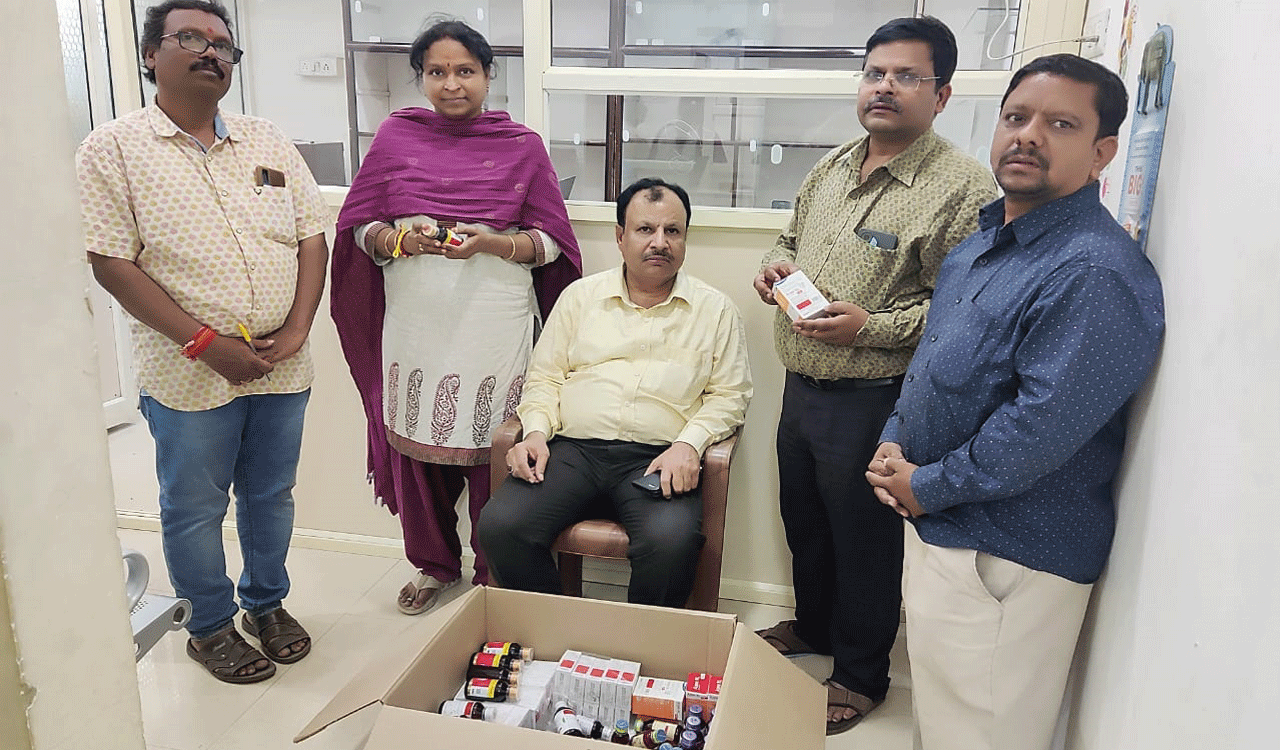
In separate developments, the TSDCA teams conducted raids at two facilities run by unqualified medical practitioners (quacks) at Chintal and Sangareddy district.
At Chintal, Quthbullapur village, Medchal-Malkajgiri, the DCA teams raided the facility of P Ravindran, who was practicing medicine without any qualification and seized 22 varieties of drugs while at Fasalwadi village, Sangareddy district, the DCA teams raided the facility of quack Devasoth Gopal and seized 21 varieties of drugs totaling up to Rs 40, 000.
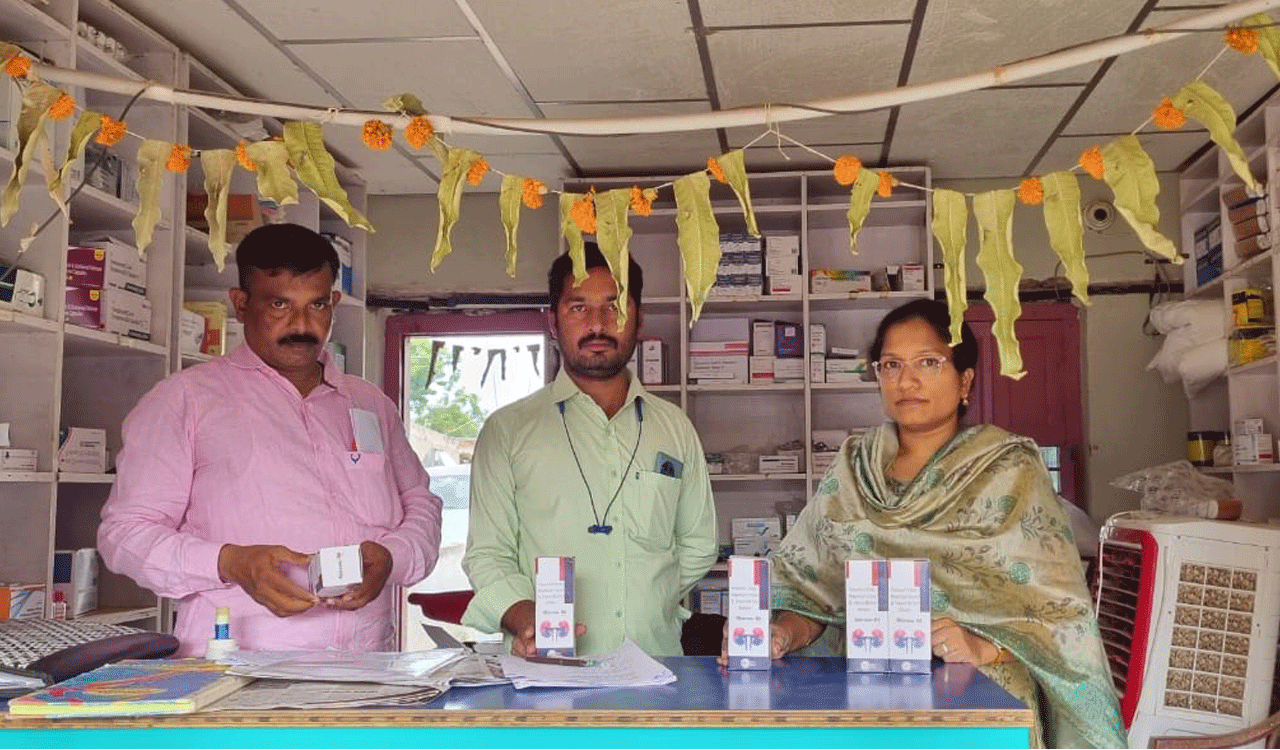
In another development, the DCA teams during raids detected Thioget Tablets (Thiamine Hydrochloride 100 mg tablets), manufactured by Lakra Polytex, Sirmour, Himachal Pradesh, that was circulating in the market.
The product (Thioget Tablets) was falsely manufactured under a ‘food license (FSSAI license)’ and falsely claimed to be a food product/nutraceutical.
According to the label composition of the product, it is classified as a drug under the Drugs and Cosmetics Act, 1940. The product must be manufactured only under a ‘drug licence’ issued under the Drugs and Cosmetics Act, adhering strictly to the ‘Good Manufacturing Practices’ (GMP) outlined in Schedule-M of the Drugs Rules.
Additionally, it must meet the quality standards prescribed in the ‘Indian Pharmacopoeia’ (IP) as mandated, the DCA press release said.
Related News
-
This is taxpayers’ money: Supreme Court raps freebies culture
10 mins ago -
Hyderabad: Residents oppose Gandhi Sarovar Project over ‘forcible’ land acquisition
22 mins ago -
Australia level series as Indian women slide to 19-run defeat in second T20I
36 mins ago -
Karnataka beat Uttarakhand in semis, to face Jammu and Kashmir in Ranji final
41 mins ago -
Five Osmania varsity players in South Zone squad for Vizzy Trophy
49 mins ago -
Disciplined West Indies bundle out Italy with ease, tops Group C in T20 WC
51 mins ago -
Zimbabwe shock Sri Lanka to enter super eights, African team’s sublime run continues
52 mins ago -
ACB finds irregularities during surprise check at Dundigal municipal office
1 hour ago