Bharat Biotech’s Covid nasal vaccine gets CDSCO approval in India
Bharat Biotech on Monday announced that its iNCOVACC, its Covid vaccine in the form of nasal drops, has received approval from the CDSCO
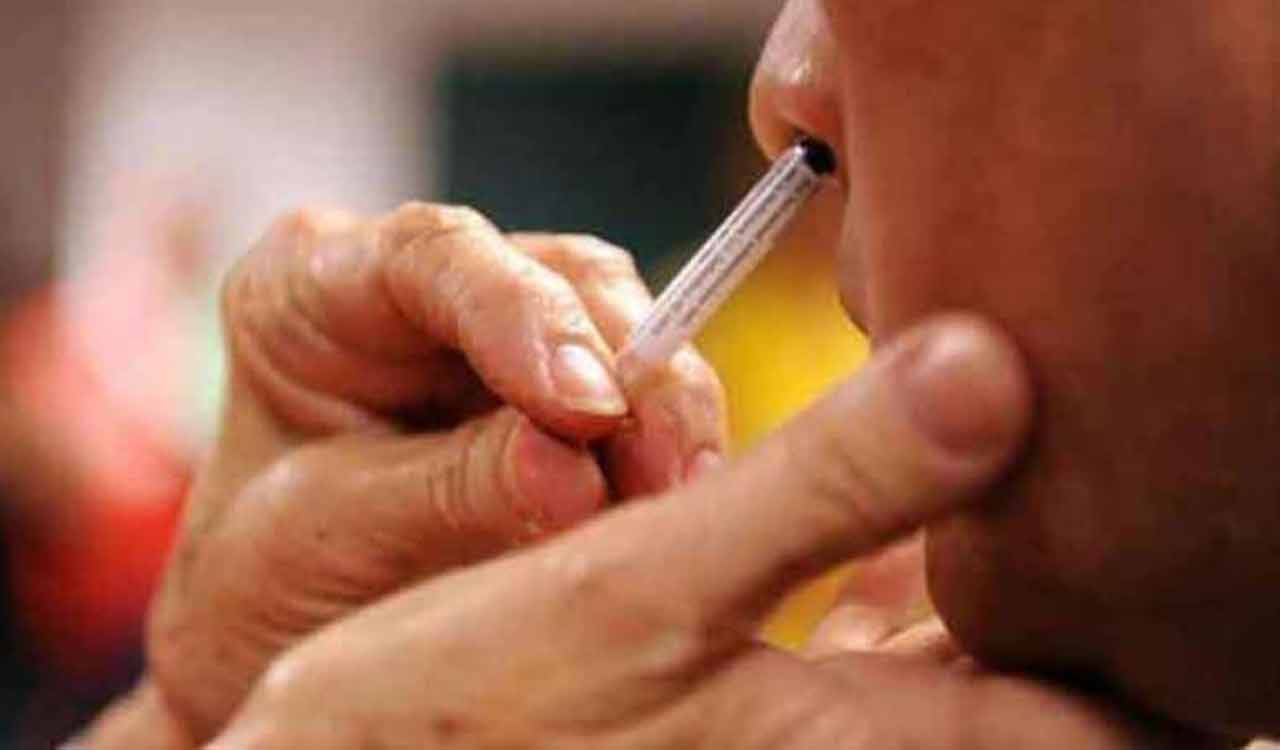
Hyderabad: Hyderabad-based global vaccine maker Bharat Biotech International Limited (BBIL) on Monday announced that its iNCOVACC (BBV154), its Covid vaccine in the form of nasal drops, has received approval from the Central Drugs Standard Control Organisation (CDSCO) under Restricted Use in Emergency Situation for ages 18 and above, in India, for heterologous booster doses.
This vaccine candidate was evaluated in phases I, II and III clinical trials with successful results. The nasal delivery system has been designed and developed to be cost-effective in low- and middle-income countries. iNCOVACC was developed in partnership with Washington University, St. Louis, which had designed and developed the recombinant adenoviral vectored construct and evaluated in preclinical studies for efficacy.
Also Read
Product development related to preclinical safety evaluation, large-scale manufacturing scale up, formulation and delivery device development, including human clinical trials were conducted by Bharat Biotech. Product development and clinical trials were funded in part by the Government of India, through the Department of Biotechnology’s, COVID Suraksha Program.
“iNCOVACC is an intranasal vaccine for the primary 2-dose schedule, and heterologous booster dose. This is a great achievement for us and the global scientific community to enable nasal administration of Covid vaccines. Despite the lack of demand for Covid vaccines, we continued product development in intranasal vaccines to ensure that we are well-prepared with platform technologies for future infectious diseases.
iNCOVACC has been designed for efficient distribution, easy and pain-free administration. We have also initiated development of variant-specific vaccines for Covid for future preparedness,” said Dr Krishna Ella, Chairman and Managing Director, Bharat Biotech.
Clinical trials were conducted to evaluate iNCOVACC as a primary dose schedule, and as heterologous booster dose for subjects who have previously received two doses of the two commonly administered Covid vaccines in India.
Immunogenicity was evaluated through the Plaque Reduction Neutralisation Tests (PRNT) and Immunoglobulin G (IgG) assays. PRNT uses the ability of a specific antibody to neutralise a virus, in turn, preventing the virus from causing the formation of plaques in a cell. IgG is the most common antibody in blood and other body fluids, and protects against bacterial and viral infections. IgG can take time to form after an infection or immunisation.
“DBT is fostering biotech enterprises and innovation ecosystems and strengthening Indian bioeconomy. DBT, along with BIRAC, is dedicated to the development of effective and safe Covid-19 vaccines under Mission Covid Suraksha. The DCGI’s approval of Bharat Biotech’s intranasal vaccine iNCOVACC is a moment of great pride for our country. This move will further strengthen our collective fight against the pandemic and broaden vaccine coverage,” said Dr Rajesh S Gokhale, Secretary, Department of Biotechnology and Chairperson of Biotechnology Industry Research Assistance Council.
“We are excited by the expansion of the Emergency Use Authorisation for iNCOVACC as a booster, which enables this intranasal vaccine to be used by many more people, and hopefully curtail transmission. This approval will increase the options for people to get vaccinated and protected against the SARS-CoV-2 virus during the ongoing pandemic,” said Michael S Diamond, of Washington University in St Louis, who co-developed the nasal vaccine technology with Washington University colleague David Curiel.
Washington University licensed the vaccine technology to Bharat Biotech in 2020 for further development. iNCOVACC is stable at 2-8°C for easy storage and distribution. Bharat Biotech has established large manufacturing capabilities at multiple sites across India, including Gujarat, Karnataka, Maharashtra and Telangana, with operations pan India.
Related News
-
Telangana HC halts Ibrahimpatnam Municipal Council election process
2 mins ago -
Telangana government cuts fees for private engineering colleges
5 mins ago -
BRS leaders approach SEC seeking action against Congress atrocities in civic polls
7 mins ago -
Girls to get location benefit in Telangana EAPCET centre allotments
16 mins ago -
Man murdered by wife for forcing her into prostitution in Mancherial
27 mins ago -
Burgampad police arrests two for attempting to kill their friend to claim insurance money
32 mins ago -
Married woman attacked by widower in Mahabubabad with petrol
35 mins ago -
Medak District Collector sentenced to six months in jail
41 mins ago